November 7, 2020
24-Month Outcomes from the DETOUR 1 Trial for Percutaneous Femoropopliteal Bypass
Presented by Ehrin Armstrong, MD
The DETOUR1 Trial was designed to evaluate the safety and effectiveness of the Detour System for percutaneous fem-pop bypass, which received FDA’s Breakthrough Device Designation. Using the novel PQ Crossing Device and a series of specially designed Torus Stent Grafts, the Detour procedure was created to route blood flow around severe lesions caused by complex peripheral artery disease found in the SFA. The procedure uses the femoral vein as a pathway for a Torus stent graft conduit and travels from the artery to the vein and back into the artery.
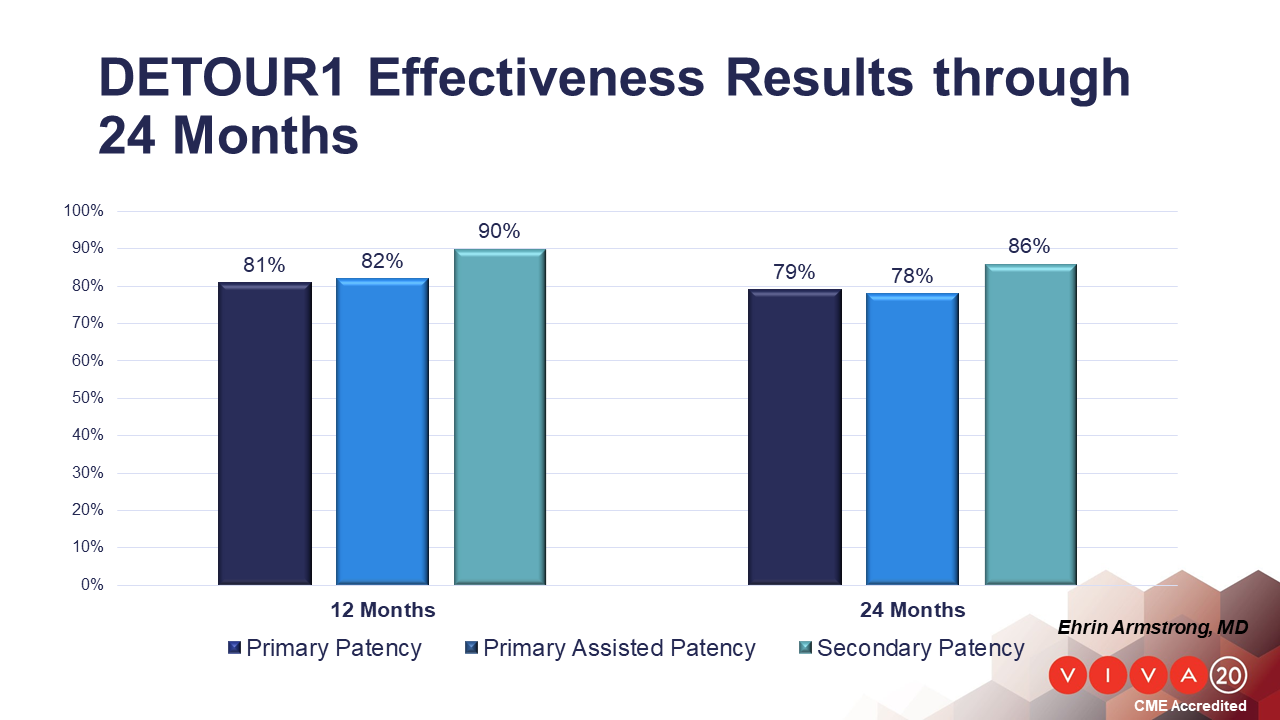
Now reporting 24-month data, the trial enrolled an extremely complex patient cohort with an average lesion length of 371 mm; 96% of lesions had a confirmed CTO and 80% had evidence of moderate to severe calcification. At 12 and 24 months post-procedure, the study is reporting an 81% and 79% primary patency rate, respectively, as well as a rate of freedom from major adverse events of 83.7% and 82.1% at 12 and 24 months, respectively.
About VIVA Physicians
VIVA Physicians, a not-for-profit organization dedicated to advancing the field of vascular medicine and intervention through education and research, strives to be the premier educator in the field. Our team of specialists in vascular medicine, interventional cardiology, interventional radiology, and vascular surgery is driven by the passion to advance the field and improve patient outcomes. Educational events presented by VIVA Physicians have a distinct spirit of collegiality attained by synergizing collective talents to promote awareness and innovative therapeutic options for vascular disease worldwide. To learn more about VIVA Physicians, visit www.vivaphysicians.org.
SOURCE VIVA Physicians
For further information: press@vivaphysicians.org
